What is PWS (Pharmaceutical Water System)?
Water is an essential ingredient to pharmaceutical manufacturing. Once purified, non-compendial water, purified water or water for injection must be stored and distributed in systems appropriately designed, installed, commissioned and validated. The water used in the pharmaceutical industry must be in optimal and standard conditions from a microbial and chemical point of view, so that it can be used in the production process and other daily uses in the pharmaceutical factories.The water machine usually consists of several parts, which include all or some of the following:

The choice of the type and device of the water maker is directly related to the existing requirements (URS) and its use. For example, the water for injection (WFI) used to produce injectable products must have the highest degree of purity and quality.
Types of water used in the pharmaceutical industry
Pure water (Purified Water - PW)
This type of water is obtained from drinking water and is used for washing devices and environments for making non-sterile products or for use in making non-injectable products.
Water for Injection (Water for Injection - WFI)
This water is used for injection purposes and making injection products and washing special devices for making injection products. The production system of this type of water should minimize the level of microbial contamination.
Highly Purified Water (HPW)
This type of water is very pure and is used to produce pharmaceutical products that require high biological quality. This water is not used for injection.
Pure Steam
This steam is used to sterilize tools and equipment and must be produced from PW or WFI water to remove contaminants from the surface of the tool.
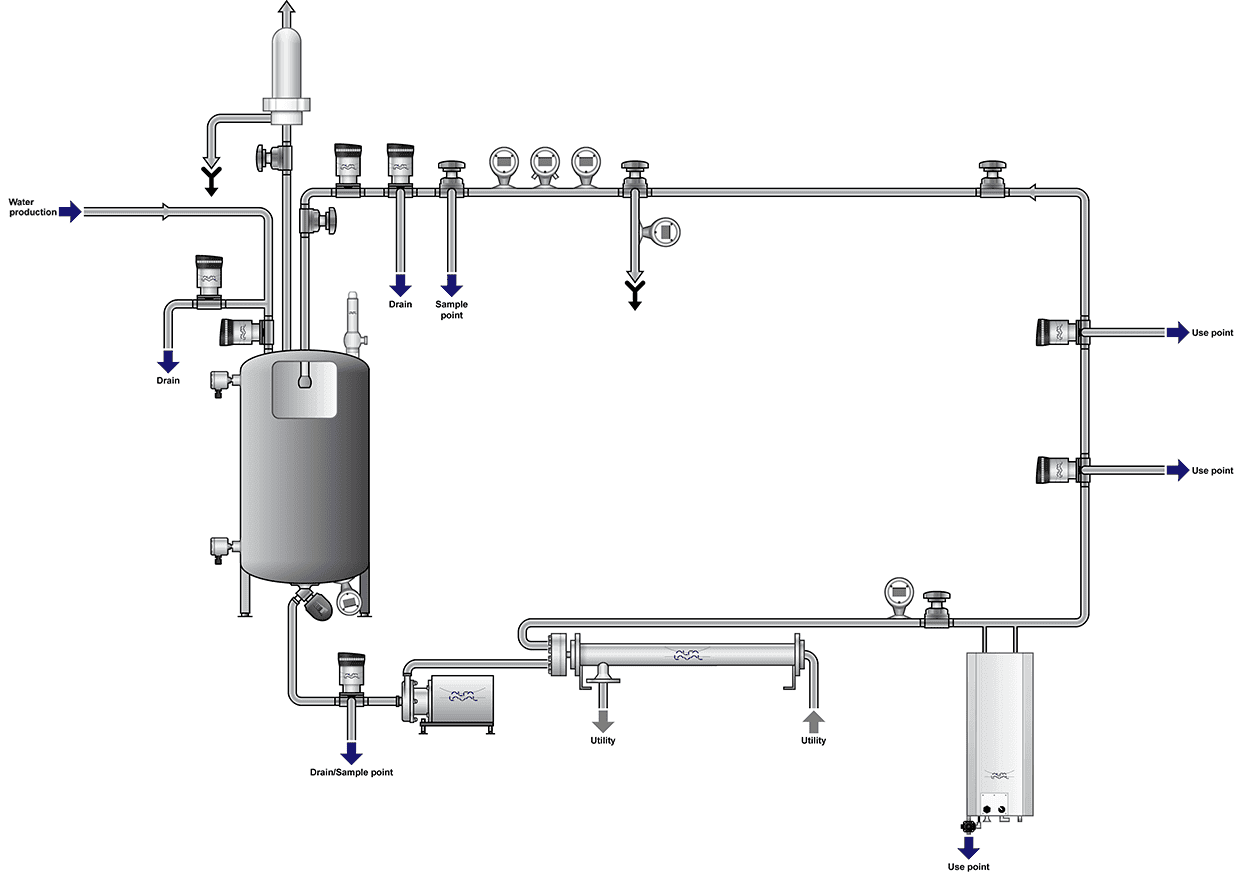
Important parameters in pharmaceutical water quality
In the pharmaceutical industry, important and vital parameters are continuously monitored to ensure the quality of water. Some parameters to monitor water quality include:
Conductivity It measures the ability of water to conduct electricity, which indicates the presence of ions. Lower conductivity means higher purity.
Total Organic Carbon (TOC) This parameter measures the amount of organic carbon in water, which can indicate pollution by organic matter.
Bioburden This parameter refers to the number of living microorganisms in water. To prevent contamination, it is necessary to keep the bioburden level low.
Dissolved Ozone Ozone is used in medicinal water production processes due to its antimicrobial properties, and the concentration of ozone must be under control.
Gito solution for PWS
It is an integrated software solution from Gito Ecosystem sub-programs that can be used for continuous and online monitoring of water generators in pharmaceutical factories and medical equipment. This software is connected to the PLC through the LAN port (Ethernet) and collects all the data under the control of this PLC and stores it in the database. After this step, all data will be displayed in a web-based and graphic form, and users can receive additional and analytical reports from the stored data. These reports include detailed graphs and tables with the ability to filter data. With the help of external modules, monitoring software can record deviations and intercept up to the CAPA stage and close the case, and it also has the ability to create voice, SMS and email alerts, which are very useful in monitoring the system in real-time.